Learning about your genome has been crucial now, more than ever. From population studies to precision diagnostics and experimental therapies, genomics is paving the way for modern medicine. Ground-breaking? Absolutely. Beneficial to us all? Perhaps not as much as we think so. By Nitish Aswani.
Your life is a story spelled out with your DNA. The most interesting facts that uniquely belong to you are wrapped up in the strands of your genes. How these genes can “solve the missing jigsaw piece for human medicine is an exciting future to look forward to,” says Dr Mark Kristansen, Head of Genomics at the Zayed Centre for Research into Rare Diseases in Children.
The leap from two decades ago, when the scientific community sequenced the first genome, to the present has been made largely possible by the advent of high-throughput sequencing technologies. The majority of genomic technologies analyse an individual’s genome by comparing it to a reference sequence, identifying patterns - especially irregularities - and predicting a phenotype (trait) based on existing studies and literature that establish a causal link between the mutation and the very trait [1]. This information feeds into hundreds of precision medicine pipelines, such as diagnostic and biomarker tests, population studies and life-changing gene therapies. Of course, without genomic data in the first place, these therapies have close to no chance of reaching patients, which stresses the importance of wide-scale sample collection.
Unfortunately, the current landscape falls short of this objective, as most genetic studies are based on populations of European ancestry. As a result, the potential benefits of genomic research—including a better understanding of disease aetiology, early detection and diagnosis, rational drug design and improved clinical care—may elude the many underrepresented populations [2].
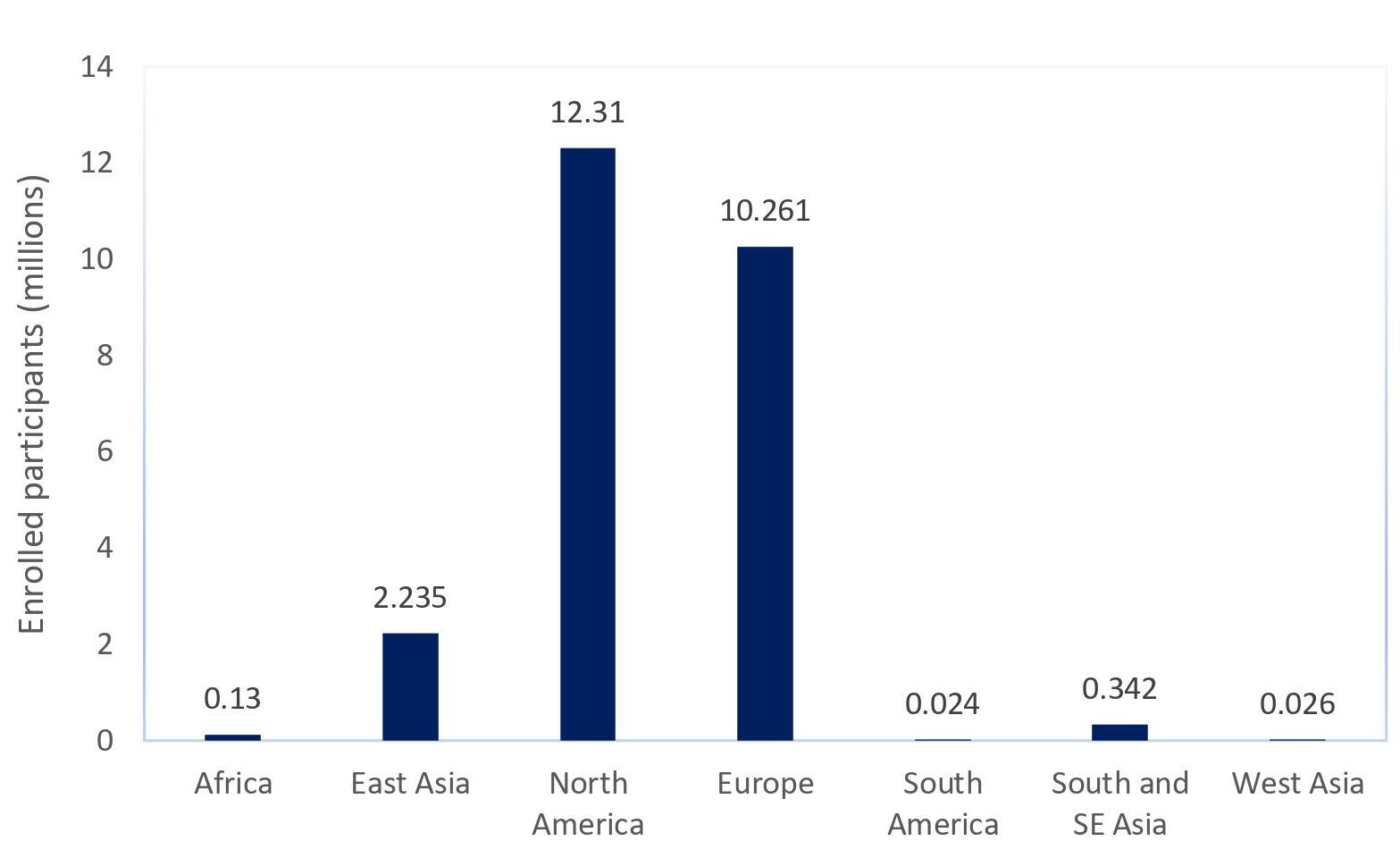
Eurocentric biases in genetics studies are largely driven by ethical issues, including inequitable resource allocation and potential healthcare disparities stemming from imbalanced research. The most significant bottleneck in establishing ethnically unbiased biobanks is the lack of comprehensive educational and awareness programs in underrepresented regions, leading to scepticism in the public [3]. This is best exemplified using a case study such as Qatar, a gravely underrepresented nation despite its modern systems.
Qatar is often seen as a symbol of ethnic diversity, a melting pot described as the “cornerstone of migration and civilisation” by Dr Younes Mokrab, Head of the Medical and Population Genomics Lab at Sidra Medicine in Qatar. In fact, an estimated 15% of the population is comprised of non-native Arabs [4]. Hence, Qatar is an ideal landscape to conduct genomic testing. Furthermore, Qatar’s funding towards infrastructure projects, such as the Sidra Medicine institute itself, demonstrate its capacity to fund medical research projects. How, then, does a country with the infrastructural and technological capabilities of introducing such powerful technology fall so far back? The answer, according to Dr Kristiansen, lies in Qatar’s historical roots.
Qatar was a once nation engulfed by vast desert land, lacked proper infrastructure and populated with large tribal groups. Despite enormous progress in medical infrastructure, there remained a lurking social stigma and fear of wanting to find out about personal genomics. Genomic profiles were known to even lead to marriages falling out, as the revelation of a potential late-onset, life-threatening disorders could negatively impact families and their reputation in society, along with detriment to ancestries. Unfortunately, these mindsets remain prevalent for many modern Qataris [3]. Contrary to assumption, this is likely not due to the poor integration of genomic technology, as the same sequencing and analytic platforms are being used to solve major problems in agriculture and microbiology. Rather, “the hurdle lies in encouraging and building trust amongst individuals through the means of education,” emphasises Dr Kristiansen. This is also exacerbated by political tensions, economic crises and regional or social conflicts in the Middle East, which adds complexity to governments’ resource allocation strategies [3].
What should be done about this? Firstly, it is vital that trained genetic counsellors - an underrepresented profession in Qatar and the wider Middle East - are employed to mitigate social stigmas around genetics [3]. Healthcare professionals are urged to educate decision-makers about the long-term societal and economic burdens of genetic diseases, so as to invest in relevant genomic research and training programs. Outcomes of such programs include training local professionals in genomics, and building the genetic evidence to guide public health efforts, including national genetic screening programs, to be implemented in healthcare systems [3]. Furthermore, various authorities must contribute to improving education technologies, developing online platforms like courses, apps, or international symposiums that facilitate the remote transfer of knowledge. Dr Kristiansen’s recent efforts organising the U.A.E Aspiring Medics Conference achieved just this, alongside a team of renowned scientists inspiring thousands of students about the prospects of genomic technologies [5]. Additionally, he leads education projects in Abu Dhabi and Hong Kong, where teams of 80 enthusiasts educate the public on the social implications of genomics research, placing emphasis on older demographics as they tend to be more reserved and sceptical.
This paradigm shift has also emerged in European nations, with the UK leading from the front. Companies like Genomics England [6] and charities like Our Future Health [7] are partnering with the NHS to spread awareness, collect samples and build large, ethnically diverse biobanks to drive clinical solutions. Furthermore, companies like AstraZeneca, GSK and IQVIA are harnessing blockchain-based big data systems of Amazon Web Services to decentralise and encrypt genomic data, thereby providing extra layers of security in data ownership and sharing [8]. These efforts have only recently been actioned, with tremendous support from industry and charitable organisations paving the way for the future of personalised healthcare. Hence, the question is whether you will join forces in this movement.
Bibliography
- Fatumo S, Chikowore T, Choudhury A, Ayub M, Martin AR, Kuchenbaecker K. A roadmap to increase diversity in genomic studies. Nature Medicine. 2022 Feb 1;28(2):243–50.
- Razali RM, Rodriguez-Flores J, Ghorbani M, Naeem H, Aamer W, Aliyev E, et al. Thousands of Qatari genomes inform human migration history and improve imputation of Arab haplotypes. Nature Communications. 2021 Oct 12;12(1).
- Abou Tayoun AN, Rehm HL. Genetic variation in the Middle East—an opportunity to advance the human genetics field. Genome Medicine. 2020 Dec;12(1).
- Qatar Population and Expat Nationalities [Internet]. Onlineqatar.com. 2019. Available from: https://www.onlineqatar.com/visiting/tourist-information/qatar-population-and-expat-nationalities
- UAE Conference [Internet]. Medefine. Available from: https://www.medefine.org/uae-conference
- Genomics England [Internet]. Genomics England. 2019. Available from: https://www.genomicsengland.co.uk/
- Our Future Health [Internet]. ourfuturehealth.org.uk. Available from: https://ourfuturehealth.org.uk/
- Jin X-L, Zhang M, Zhou Z, Yu X. Application of a Blockchain Platform to Manage and Secure Personal Genomic Data: A Case Study of LifeCODE.ai in China. Journal of Medical Internet Research. 2019 Sep 10; 21(9).