Currently, there are limited clinical treatment options to deal with cases of synthetic cannabinoid abuse. A novel approach utilising nanoparticle vaccines offers an unconventional solution, but not without challenges to its clinical application. By James Lau Yi Wei.
In the last decade, Synthetic Cannabinoids (SCs) have become widely commercially available in health food shops and on the internet. They are often marketed as herbal remedies with therapeutic benefits, but in reality, SCs are mind-altering drugs that can have dangerous effects. Consumption has resulted in cases of cardiotoxicity, severe psychosis and even death [1]. Specific treatment options for SCs are outcompeted by the speed with which suppliers introduce new and increasingly dangerous synthetic iterations to the market. Current treatment options such as using intravenous fluids, benzodiazepines or anti-emetics only provide temporary relief and do not remove SCs from the body [2].
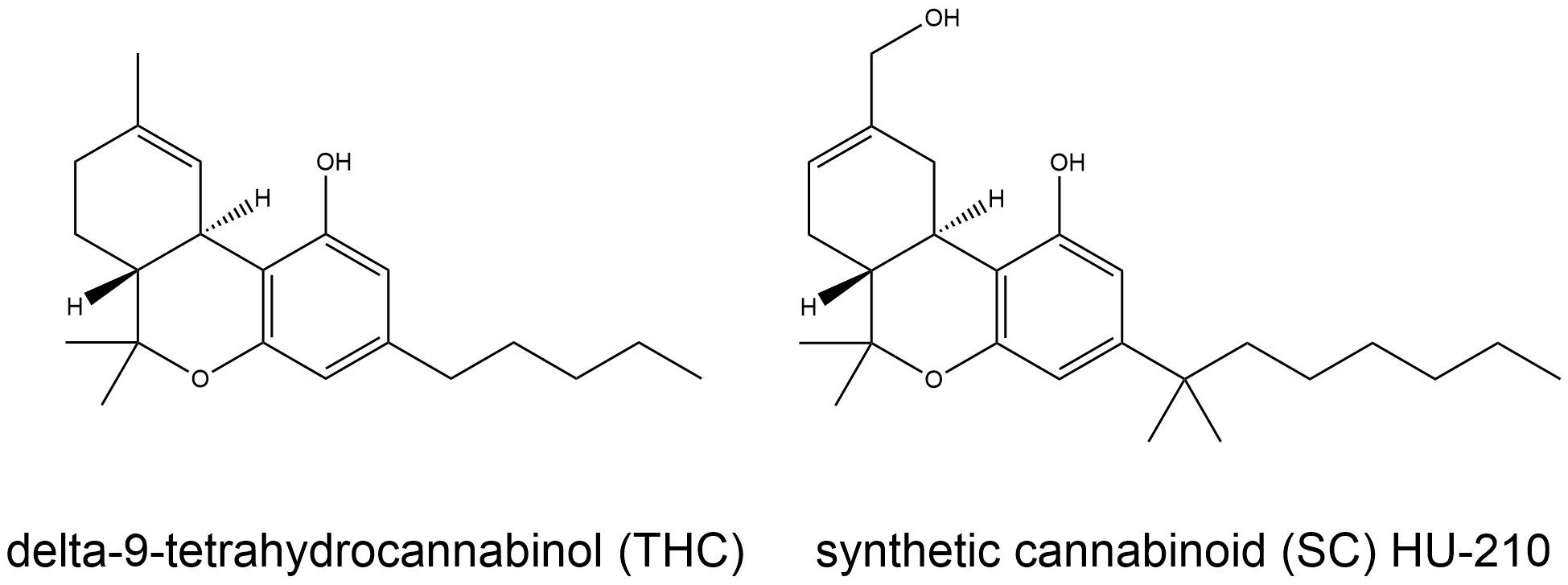
SCs were initially designed as analogues to the naturally occurring Δ9-tetrahydrocannabinol (Δ9-THC) molecule - free from its psychoactive properties and armed with beneficial medicinal effects [3]. Both SCs and Δ9-THC have a similar mechanism of action: they bind to the cannabinoid receptor CB1, which is found in the central and peripheral nervous system. As full agonists, SCs have greater binding affinities to the CB1 receptor compared to Δ9-THC, causing increased inhibition of nerve impulses [4]. In mice, this was observed to cause catalepsy and symptoms such as reduced movement, hypothermia and diminished pain sensitivity [3]. Ultimately, the risk of requiring emergency medical treatment following the use of synthetic cannabinoids was estimated to be 14 to 30 times greater compared to the use of traditional cannabis [2]. Hence, the dangers associated with SCs underscores a desperate need for a new type of treatment that can neutralise the drug in the body before it can bind to the CB1 receptors.
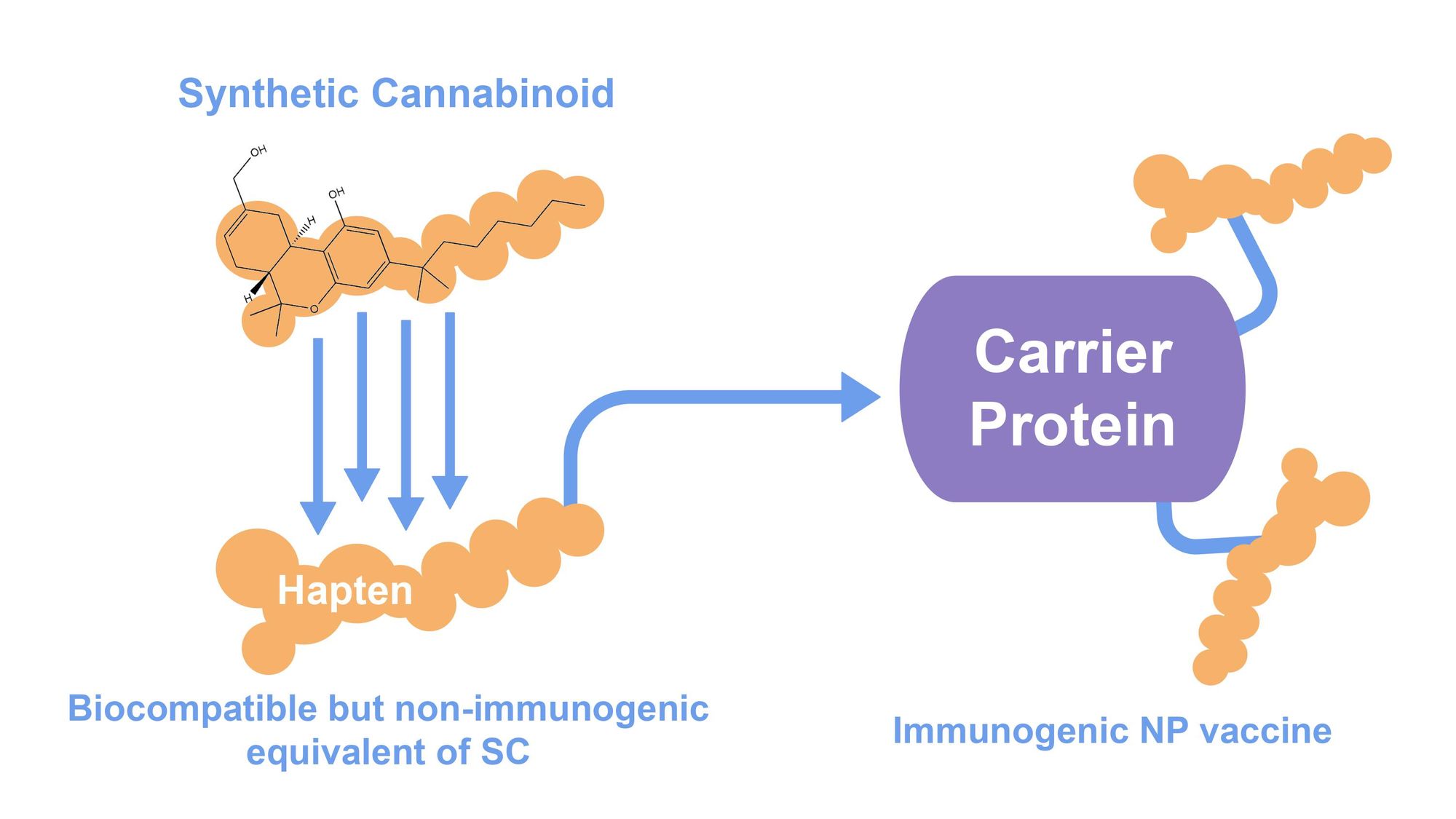
Experimental nanoparticle (NP) vaccines could potentially protect against harmful SCs via an immune response - a treatment similar to flu vaccines. This emerging solution is unique in that it could provide immunisation against foreign bodies that do not contain proteins, while being too small in size to elicit an immune response themselves. NP vaccines achieve this due to the functionality of its two components, the most important of which is hapten. Haptens have a chemical structure similar to that of the SC antigen. This enables B-cell recognition of the foreign SC when it appears in the bloodstream, stimulating the production of neutralising antibodies. As haptens themselves are unable to activate the immune system, they must be attached to an immunogenic protein carrier, which is derived from molluscs or cows [1]. Vaccination will elicit a subsequent antibody response that will neutralise the target SCs in the bloodstream before it can bind to its receptor target to cause psychotropic effects [5].
Another potential benefit of using NP vaccines against SCs is that they might be able to reverse symptoms associated with SC overdose. This was demonstrated in a recent study in which immunised mice averted catalepsy and associated symptoms of SC toxicity [1].
The increasing abundance of SCs as novel psychotic substances, with more than 260 derivatives in circulation, present a significant challenge to clinical translation of NP vaccines [1]. One of the underpinning principles of drug design is site selectivity, which means that drug molecules can be precisely designed to bind to a specific target molecule. There are already hundreds of different individual SC molecules in existence, with more to come that will certainly circumvent drug tests. As such, it will be immensely difficult to design a hapten that will enable the immune system to identify and elicit an immune response to new and unknown SCs, while maintaining affinity for existing ones.
An initial approach to this challenge could be to investigate an assay of multiple SCs with their hapten derivatives to test their immune response. This may help us understand the relationship between antibody recognition of SCs and hapten chemical structure. Hopefully, this data can illuminate the most effective SC derivative to prepare more successful NP vaccines.
References:
[1] Worob, A.; Wenthur, C.J. Development of Cross-Reactive Antibodies for the Identification and Treatment of Synthetic Cannabinoid Receptor Agonist Toxicity. Vaccines 2022,10,1253. [Accessed on: 28/11/2022] Available from: https://doi.org/10.3390/vaccines10081253.
[2] Robert J. Tait, David Caldicott, David Mountain, Simon L. Hill & Simon Lenton (2016) A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment, Clinical Toxicology, 54:1, 1-13. [Accessed on:4/12/2022] Available from: http://dx.doi.org/10.3109/15563650.2015.1110590.
[3] Schreiber, S., Bader, M., Lenchinski, T., Meningher, I., Rubovitch, V., Katz, Y., Cohen, E., Gabet, Y., Rotenberg, M., Wolf, E. U., & Pick, C. G. (2019). Functional effects of synthetic cannabinoids versus Δ9 -THC in mice on body temperature, nociceptive threshold, anxiety, cognition, locomotor/exploratory parameters and depression. Addiction biology, 24(3), 414–425. [Accessed on: 4/12/2022] Available from: https://doi.org/10.1111/adb.12606.
[4] Walsh, K.B.; Andersen, H.K. (2020) Molecular Pharmacology of Synthetic Cannabinoids: Delineating CB1 Receptor-Mediated Cell Signaling. Int. J. Mol. Sci. 2020, 21, 6115. [Accessed on: 11/1/2022]. Available from: https://doi.org/10.3390/ijms21176115
[5] Brisse, M., Vrba, S. M., Kirk, N., Liang, Y., & Ly, H. (2020). Emerging Concepts and Technologies in Vaccine Development. Frontiers in immunology, 11, 583077. [Accessed on: 4/12/2022]. Available from: https://doi.org/10.3389/fimmu.2020.583077.